Bond Angles Of Trigonal Pyramidal
Trigonal pyramidal is a geometry of some molecules like ammonia and phosphine. Permit united states of america an example of ammonia to understand the trigonal pyramidal molecular geometry. Ammonia is an inorganic compound with three hydrogen atoms surrounding one nitrogen atom. All three hydrogen atoms shared their valence electrons with the nitrogen cantlet forming iii strong covalent bonds. The nitrogen atom in ammonia contains a lone pair of nonbonded electrons.
This pair of nonbonding electrons on nitrogen repeal the electron shared by the hydrogen atoms. Due to this repulsion, the hydrogen atom spreads out to grade a triangular base of operations with the nitrogen atoms sitting at the apex of the molecule. When the shape is drawn out information technology resembles a triangular-based pyramid with the hydrogen atoms forming the bottom base and the nitrogen atom forming the apex of the pyramid.
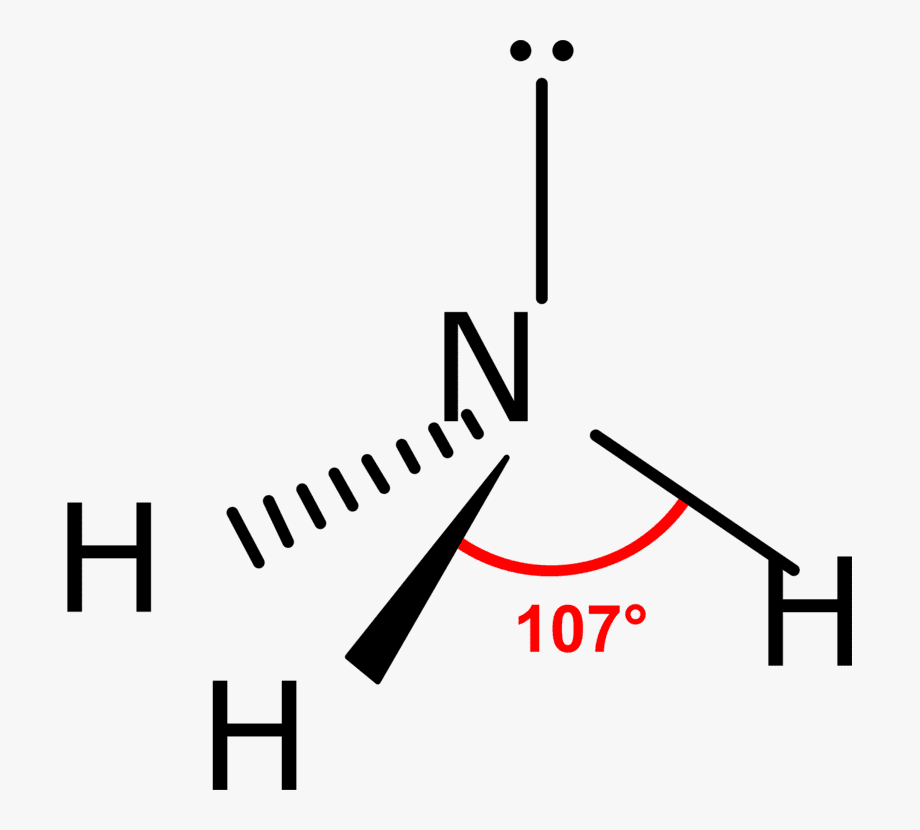
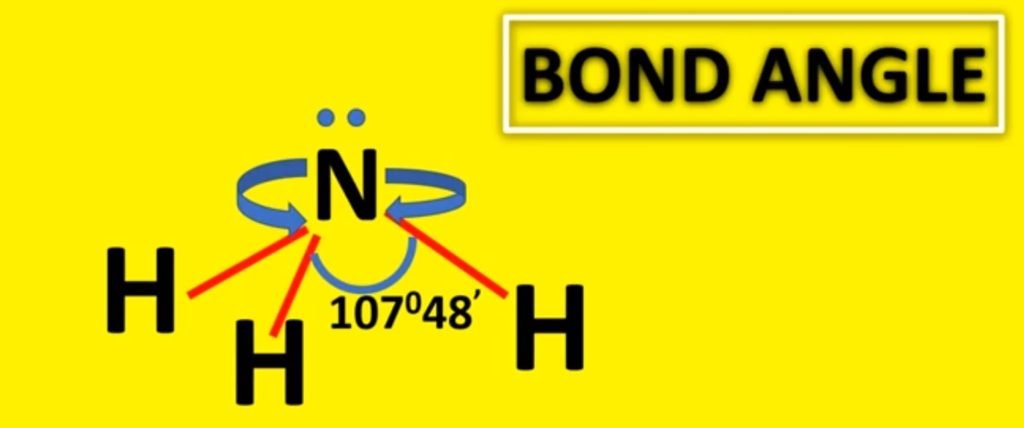
The bond bending in ammonia is approximately 107 degrees and this shape is similar to the tetrahedral shape of methyl hydride. In the sense that they can both exist drawn out at a tetrahedron. However, the difference is that the central atom in ammonia is at the apex of the tetrahedron while the key atom in methane is embedded in the tetrahedron.
Trigonal Pyramidal Geometry of Ammonia
The molecular formula of ammonia is NH3. The central atom is nitrogen having atomic number seven. The electronic configuration of nitrogen is 1s2, 2s2, 2px one 2py 1 2pz 1. The hydrogen diminutive number is 1. Its electronic configuration is 1s1. If 1s orbitals of hydrogen atoms overlap with three p orbitals of 1 nitrogen atom. There must be three p-s sigma bonds with a bond angle of 90 degrees between HNH in ammonia. But really, the bond angle is approximately 107 degrees. How does it happen?
If we find the valency electronic configuration of nitrogen, in that location is a minor energy departure between 2s and 2p orbitals.
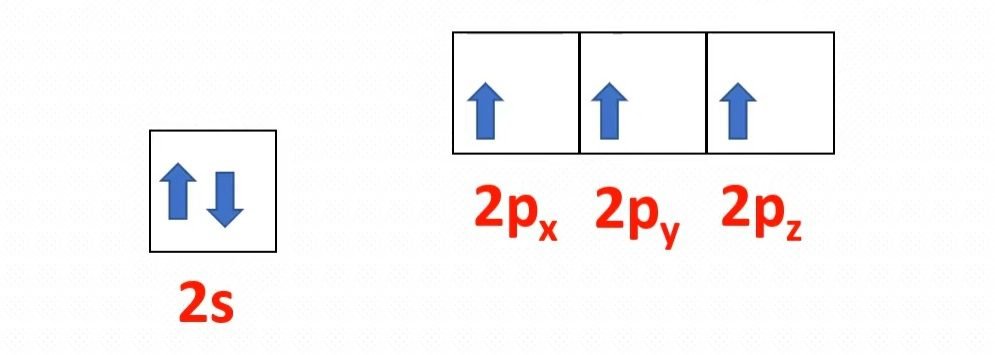
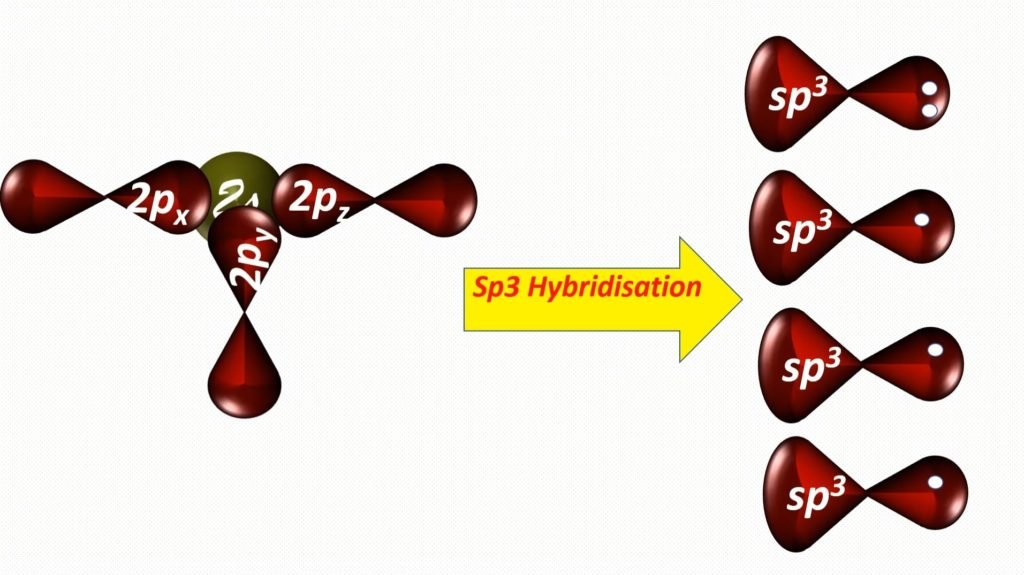
In nitrogen, between 2s and 2p orbitals, the procedure of hybridization is occur. Hybridization is a phenomenon of intermixing of outermost atomic orbital and their re-shuffling into the aforementioned number of orbitals simply with equal backdrop similar energy and shape.
After the hybridization of the central atom, the bond germination of nitrogen and hydrogen.
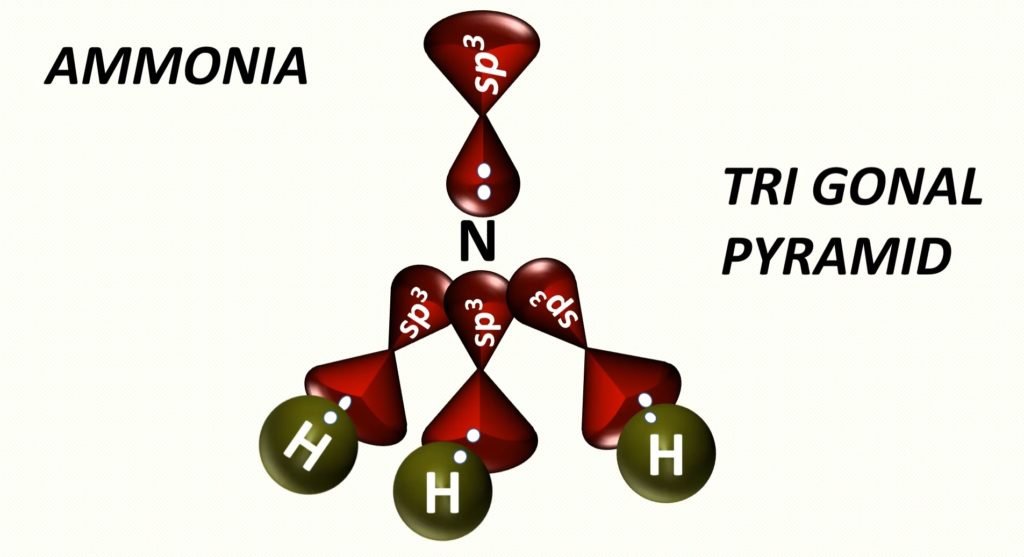
Geometry of Ammonia
Trigonal Pyramidal
Bond Angle of Ammonia
Due to sp3 hybridization, its bond bending must be 109°28′ but due to the repulsion of the solitary pair and bond pair, its bond bending is 107°48′.
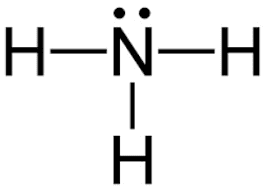
Lewis Dot Construction of Ammonia
NH3 lewis structure, first, nosotros calculate Q.
Q = Valance electron of all atom + no of -ve charge – no of +ve charge
Q = (8 + 0 – 0)
Q = 8
B.P east– = 2 × no of bonds
B.P e– = 2 × 3
B.P east– =half dozen eastward–
L.P eastward– = Q – B.P e–
L.P e– =8 – 6
50.P due east– = ii
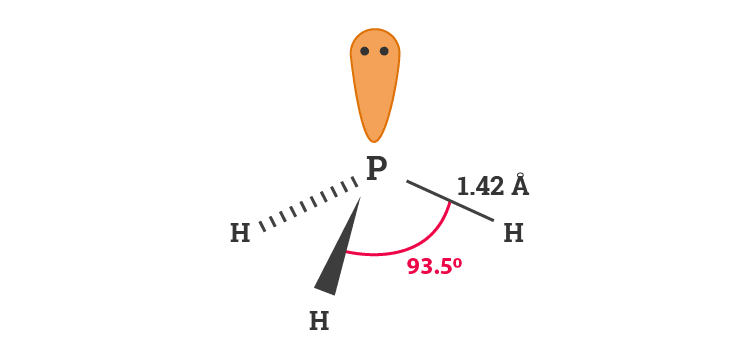
Trigonal Pyramidal Geometry of Phosphine
The molecular geometry of phosphine is similar to ammonia, trigonal pyramidal. Phosphine like ammonia has iii hydrogen atoms spread out to form a triangular base just as with ammonia. This is because of lone pair of electrons present on the central atom phosphorus in phosphine.
Summary
- Some examples of trigonal pyramidal molecules are Ammonia and phosphine.
- Trigonal pyramidal molecules take three atoms arranged in a triangular base of operations, with a fundamental atom placed at the noon forming a pyramid.
Bond Angles Of Trigonal Pyramidal,
Source: https://www.uochemists.com/trigonal-pyramidal/
Posted by: beckdiden1961.blogspot.com


0 Response to "Bond Angles Of Trigonal Pyramidal"
Post a Comment